Blog
Successful Implementation of Lipiflow into Practice
Implementation of LipiFlow® into practice: Evidence & clinical tips
Practitioner summary box
• LipiFlow is indicated for the treatment of meibomian gland dysfunction.
• LipiFlow is more effective in less severe gland disease and with a shorter duration of time between MGD diagnosis and treatment.
• Osmolarity is useful for diagnosis, but not necessary. Treatment outcomes can be monitored easily by other tests.
• The success rate with LipiFlow is 86%. It is important to counsel patients on what to expect from this treatment.
• Combining Lipiflow with warm compresses improves the treatment effect of LipiFlow.
• Weak evidence suggests that LipiFlow may improve meibomian gland atrophy. More research is needed to substantiate these findings.
• LipiFlow is safe to use and is only associated with minor discomfort during the insertion of activators in some patients.
• Symptom improvements tend to occur of 4 weeks, so ensure to advise your patients not to expect any improvements before then. It can take up to three months for the tear film to stabilise.
• LipiFlow has been shown to be effective at maintaining improved meibomian gland secretion for up to three years, however tear film stabilisation only lasts for up to one year. Symptoms improvement may last for 1-3 years.
• There is no added benefit from repeating LipiFlow within 12 months.
• LipiFlow treatment prior to cataract surgery stabilises spectacle refraction and prevents deterioration of TBUT following surgery.
What is LipiFlow®?
LipiFlow® was approved by the FDA for the treatment of MGD in 2011[1]. It uses vector thermal pulsation, at 42.5°C for 12 minutes and only requires a single treatment. It is unique in that it applies heat to both the inner and outer eyelid at the same time, on both the upper and lower lids. No other treatment on the market applies heat to the inner lid. It has been shown to be effective for up to three years[2].
Clinical Summary: LipiFlow is a device that uses thermal pulsation to treat meibomiang gland dysfunction. It is effective because it maintains an optimal heat of 42.5°C on both the outside and inside of the eyelids. It has been shown to be effective for up to three years.
When is LipiFlow® indicated?
LipiFlow® is indicated for the treatment of MGD, but which MGD patients should you recommend this treatment for? The answer to this depends on several clinical factors including the severity of disease, patient preferences and patient finances. It requires excellent communication from yourself, the practitioner, to ensure the patient understands the benefits, risks and efficacy of treatment.
Ideally, all patients with MGD should receive this treatment. However, due to the cost of the procedure, you will find that most patients who choose the treatment will be moderate to severe in their signs and symptoms. In my own clinical practice, I have found that the less severe the disease, the quicker and more impressive the results are with LipiFlow®.
Clinical summary: LipiFlow is indicated for the treatment of meibomian gland dysfunction. It is not effective for other types of dry eye.
Does meibomian gland dropout affect the success of LipiFlow® treatment?
Research shows that there is a redundancy of meibomian glands – i.e. you can function with less glands than you have (like surviving with one kidney)[3, 4]. This suggests that there must be a threshold of meibomian gland loss beyond which a lipid layer is unmaintainable by remaining meibomian glands[5]. Turnbull et al. showed that even patients with >40% gland loss can benefit from natural release of meibum from remaining meibomian glands[6].
Finis et al. recommended performing meibography ‘in every patient before treatment for better prediction of therapeutic effects’[7]. They found that patients with a meiboscore of 6 (most severe loss) had less improvement in symptoms and number of expressible glands, compared to patients with a score of 0-5 between baseline and 6 months. They also found that patients with a meiboscore of 0-4 had a significantly greater improvement in lipid layer thickness, when compared to patients with a score of 5-6. They found that LipiFlow® has no effect on meibomian gland regeneration, which means that it primarily works on existing glands and explains why patients with severe gland loss responded poorly to the treatment.
Blackie et al. found that there were several baseline patient characteristics that are significantly associated with greater improvement in meibomian gland secretion following LipiFlow® treatment. These included a shorter duration of dry eye symptoms, shorter duration of dry eye or MGD diagnosis, and a higher (less severe) baseline meibomian gland secretion score[8].
Clinical Summary: Lipiflow is more effective in less severe gland disease and with a shorter duration of time between MGD diagnosis and treatment.
Is it necessary to measure osmolarity?
Studies that have measured osmolarity in patients receiving LipiFlow® treatment have failed to show an improvement in osmolarity[9]. It is therefore useful for diagnosis, but not for monitoring treatment outcomes with this device. According to the DEWS II guidelines[10], there are other ways to confirm a diagnosis of dry eye (e.g. corneal staining, dry eye questionnaire, tear break up time), so the answer is – no, you don’t need to measure osmolarity if you don’t have access to it.
Clinical summary: Osmolarity is useful for diagnosis, but not necessary. Treatment outcomes can be monitored easily by other tests.
What is the success rate?
Blackie et al. had a useful study design that gave participants the option of having a second treatment if they felt their symptoms were still not adequately improved[8]. At 6 months total of 94.8% of patients that received one LipiFlow® treatment chose to not have any further treatment This number reduced to 87.2% at 9 months, and 86.2% at 12 months.
Finis et al. showed the 76% of patients had an improvement of OSDI scores after LipiFlow® treatment[9]. At six months, 73.% showed a reduction of the OSDI score[7] with an average improvement from 42 to 33. Is 33 enough? It depends. 33 is the lower limit of severe symptoms on the OSDI questionnaire:
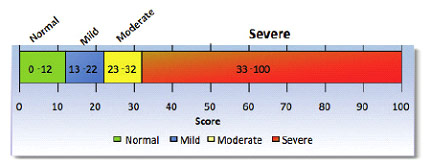
Just because patients have a significant improvement, doesn’t mean that the improvement is satisfactory for the patient. Some patients maybe happy with the improvement, others may not.
It is important to note here that an improvement does not necessarily translate to success in your patient’s eyes. It comes down to the patient’s expectations and if the improvement they’ve experienced is what they were expecting. Hence managing those expectations will be key to treatment success.
Clinical summary: The success rate with LipiFlow is 86%. It is important to counsel patients on what to expect from this treatment.
Combining LipiFlow® with conventional treatment
I recommend home lid hygiene treatment to all my dry eye patients, regardless of what in-office treatment they are receiving. This is important as it emphasises to the patient that the in-office treatment is not a final cure, but part of the overall treatment regime. I also find that patients that maintain lid hygiene have better treatment outcomes and require less frequent re-treatment with in-office procedures.
There is also evidence that shows that there is an added benefit of combining LipiFlow® with warm compresses. Finis et al showed that the crossover group, that received both LipiFlow® and warm compresses, had better OSDI scores and lipid layer thickness than the LipiFlow® only group[9].
Clinical summary: Combining Lipiflow with warm compresses improves the treatment effect of LipiFlow.
Can LipiFlow® improve MG dropout?
A study by Finis et al. showed no improvement in meibomian gland dropout over a six month follow up period[7]. Blackie et al. also showed no improvement in gland dropout at one month and three months post treatment with LipiFlow®. Akon et al., on the other hand, showed an improvement in dropout within one month of treatment[11], however there were issues with the design of this study so its results must be looked at cautiously. A case report by Korb and Blackie in 2013 also showed improvement in meibobian gland dropout.
The evidence against a therapeutic effect of LipiFlow® on meibomian gland atrophy is stronger that the evidence for its effect. More research is needed in this area as most of the available LipiFlow® studies did not perform meibography before and after treatment, and the studies that have showed improvements[11, 12] are considered low quality evidence.
Clinical summary: Weak evidence suggests that LipiFlow may improve meibomian gland atrophy. More research is needed to substantiate these findings.
Possible adverse events
A recent study reported ‘no serious, device-related, adverse events’ with LipiFlow® treatment. Minor adverse events included eye pain or discomfort or pain, which occurred in ‘patients with small eyes, narrow palpebral fissure, or deep-set eyes’[13].
This has also been my experience. As the LipiFlow® activators only come in one size, it requires practice to learn how to insert them into smaller, or deep-set, eyes. A lack of finesse, or gentleness, during insertion can cause discomfort to the patient.
Clinical Summary: LipiFlow is safe to use and is only associated with minor discomfort during the insertion of activators in some patients.
When can you expect improvements?
Li et al. showed significant improvements within 4 weeks for OSDI, NITBUT & LLT (among other measurements) within 4 weeks of treatment for both evaporative and mixed dry eye groups [14]. These improvements persisted at 8 and 12 weeks in the evaporative dry eye group, but only OSDI persisted to 12 weeks in the mixed dry eye group. Hagen et al. and Blackie et al. also showed improvements in TBUT and OSDI between baseline and three months (measurements were not taken earlier than three months in these two studies)[15, 16].
A meta-analysis that looked at four studies published between 2012 and 2016 found that LipiFlow® significantly improved meibomian gland function, tear break up time and lipid layer thickness at two to four weeks[17].
Clinical Summary: Symptom improvements tend to occur of 4 weeks, so ensure to advise your patients not to expect any improvements before then. It can take up to three months for the tear film to stabilise.
How long does the LipiFlow® treatment effect last for?
A major strength that the LipiFlow® treatment has is not only the large number of randomised control trials that have studied it, but also the length of follow up in some of these studies. These studies allow us to see how the treatment effect with the device lasts for.
Finis et al. found that subjective symptoms, lipid layer thickness, number of expressible glands, lid margin parallel conjunctival folds, and bulbar redness were all improved 6 months after treatment[7].
Blackie et al. found that at 12 months, 86% of the treatment group felt that they did not require any further treatment[8]. This group sustained a mean improvement in meibomian gland secretion from 6.4±3.7 to 17.3±9.1 (P,0.0001) and dry eye symptoms (OSDI) from 44.1±20.4 to 21.6±21.3 (P,0.0001) between baseline and 12 months.
Greiner et al. also conducted a year-long study[18]. They found meibomian gland secretion, SPEED and OSDI scores improved significantly at one month, and these improvements were maintained at one year. Tear break-up time was also significantly increased at one month; however, this improvement was no longer evident at one year post-treatment.
The above study was followed up for 3 years[2]. This is the longest follow-up data available for LipiFlow®. Only SPEED and meibomian gland secretion scores had improvements that persisted at the three year mark.
Clinical summary: LipiFlow has been shown to be effective at maintaining improved meibomian gland secretion for up to three years, however tear film stabilisation only lasts for up to one year. Symptoms improvement may last for 1-3 years.
Is there any benefit to repeating LipiFlow treatment within 12 months?
The available evidence shows that there is no benefit in repeating LipiFlow® treatment within 12 months. Blackie et al. offered participants a second treatment of LipiFlow® at 6 months if they felt they needed further improvement[8]. When comparing patients who received one LipiFlow® treatment, to those who received two treatments, the study found that there was no significant difference in the mean MGS score between the two groups. In fact, the study found that the one-treatment group had a statistically significantly lower mean OSDI score at 12 months. The authors concluded that this was likely due to the higher severity at baseline in participants that requested two treatments.
Clinical Summary: There is no added benefit from repeating LipiFlow within 12 months.
LipiFlow® and refractive surgery
Lipiflow® can be used to improve visual outcome for patients undergoing intra-ocular lens replacement surgery. Zhao et al. found that LipiFlow treatment before cataract surgery is effective in alleviating blockage of meibomian glands and preventing the deterioration of TBUT following cataract surgery[19]. Matossian et al. found the LipiFlow® in a significant change in refraction prior to surgery[20]. They found that astigmatism decreased in 24% of eyes, increased in 52% of eyes and remained unchanged in just 24%.
Clinical Summary: LipiFlow treatment prior to cataract surgery stabilises spectacle refraction and prevents deterioration of TBUT following surgery.
References
1. FDA, DE NOVO CLASSIFICATION REQUEST FOR TEARSCIENCE,INC. LIPIFLOW® THERMAL PULSATION SYSTEM. 2011.
2. Greiner, J.V., Long-term (3 year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye & contact lens, 2016. 42(2): p. 99-107.
3. Craig, J., K. Blades, and S. Patel, Tear lipid layer structure and stability following expression of the meibomian glands. Ophthalmic and Physiological Optics, 1995. 15(6): p. 569-574.
4. Blackie, C.A. and D.R. Korb, Recovery time of an optimally secreting meibomian gland. Cornea, 2009. 28(3): p. 293-297.
5. Finis, D., et al., Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Current eye research, 2015. 40(10): p. 982-989.
6. Turnbull, P.R., S.L. Misra, and J.P. Craig, Comparison of treatment effect across varying severities of meibomian gland dropout. Contact Lens and Anterior Eye, 2018. 41(1): p. 88-92.
7. Finis, D., et al., Six-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophy. Cornea, 2014. 33(12): p. 1265-1270.
8. Blackie, C.A., C.A. Coleman, and E.J. Holland, The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clinical Ophthalmology (Auckland, NZ), 2016. 10: p. 1385.
9. Finis, D., et al., Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. The ocular surface, 2014. 12(2): p. 146-154.
10. Wolffsohn, J.S., et al., TFOS DEWS II diagnostic methodology report. The ocular surface, 2017. 15(3): p. 539-574.
11. Akon, M., A. Gupta, and P.K. Kayal, Pre and Post Treatment Comparison between IPL and LipiFlow for Meibomian Gland Dysfunction.
12. Korb, D.R. and C.A. Blackie, Case report: a successful LipiFlow treatment of a single case of meibomian gland dysfunction and dropout. Eye & contact lens, 2013. 39(3): p. e1-e3.
13. Booranapong, W., et al., Comparison of an Automated Thermodynamic Treatment System (LipiFlow) and Warm Compresses for the Treatment of Moderate Severity of Meibomian Gland Dysfunction. Siriraj Medical Journal, 2020. 72(1): p. 79-86.
14. Li, B., et al., Comparison of the therapeutic effect of Meibomian Thermal Pulsation LipiFlow® on obstructive and hyposecretory meibomian gland dysfunction patients. International Ophthalmology, 2020. 40(12): p. 3469-3479.
15. Blackie, C.A., et al., A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clinical Ophthalmology (Auckland, NZ), 2018. 12: p. 169.
16. Hagen, K.B., et al., Comparison of a single-dose vectored thermal pulsation procedure with a 3-month course of daily oral doxycycline for moderate-to-severe meibomian gland dysfunction. Clin Ophthalmol, 2018. 12: p. 161-168.
17. Pang, S.-P., et al., Efficacy of vectored thermal pulsation and warm compress treatments in meibomian gland dysfunction: a meta-analysis of randomized controlled trials. Cornea, 2019. 38(6): p. 690-697.
18. Greiner, J.V., Long‐term (12‐month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clinical & experimental ophthalmology, 2013. 41(6): p. 524-530.
19. Zhao, Y., et al. Preoperative Management of MGD with Vectored Thermal Pulsation before Cataract Surgery: A Prospective, Controlled Clinical Trial. in Seminars in Ophthalmology. 2021. Taylor & Francis.
20. Matossian, C., Impact of Thermal Pulsation Treatment on Astigmatism Management and Outcomes in Meibomian Gland Dysfunction Patients Undergoing Cataract Surgery. Clinical Ophthalmology (Auckland, NZ), 2020. 14: p. 2283.

